1165
Views & Citations165
Likes & Shares
Ependymoma is relatively uncommon brain malignancy in pediatric population, typically arising from the ependyma within ventricular system. Surgery in conjunction with postoperative radiotherapy represents only curative modality. However, relapses are common, originating mainly at the initial site of the disease but craniospinal seeding is frequently observed. Herein we present a case of 7 year old female child who underwent surgical resection for parieto-occipital anaplastic ependymoma at the age of 4. First postoperative MRI scans revealed residual mass in the occipital portion of the left lateral ventricle. The child underwent re-resection, received systemic chemotherapy and six months post-surgery was treated with highly conformal proton radiotherapy to a total dose of 59.4 GyE to the resection cavity and residual tumor, given in 33 daily fractions of 1.8 GyE each. Unfortunately, she experienced quick in-field relapse and subsequently underwent four resection attempts. Finally, at the age of 6 she experienced bulky recurrence in the initial site of disease, coupled with infratentorial and cortical metastases and leptomeningeal seeding with gross deposits in lumbosacral liquor space. She was treated with salvage craniospinal photon irradiation to a total dose of 30 Gy given in 12 daily fractions of 2.5 Gy each. Short-term symptom improvement was achieved however the child succumbed 9 months after due to disease progression. No MRI signs of radiotherapy-induced myelopathy were observed.
Keywords: Ependymoma, Leptomeningeal seeding, Proton radiotherapy, Photon radiotherapy, Magnetic resonance imaging, Brain necrosis
INTRODUCTION
Ependymoma is challenging and difficult to treat brain tumor typically affecting children below the age of 5. Surgery and postoperative radiotherapy are the main treatment options. Despite these curative therapies, recurrences frequently occur, leading to poor outcome [1]. Especially unfavorable subset of patients is those with local relapse coupled with leptomeningeal seeding [2]. There is no established treatment for recurrent disease, and surgery is usually combined with chemotherapy and radiotherapy. Re-irradiation to craniospinal axis is often considered given high propensity for multifocal recurrence [3]. There is special concern associated with re-irradiation in terms of late neural toxicity [4]. In this paper, we present the case of 7 year old child with recurrent and metastatic ependymoma who was initially managed with surgery and proton radiotherapy but was subsequently treated with multiple surgeries and salvage craniospinal photon radiotherapy for overt metastatic disease. Radiotherapy management of recurrent disease post primary focal proton radiotherapy is discussed, and limited literature data reviewed.
CASE REPORT
Four year old girl presented with history of progressive headache, nystagmus, vomiting and somnolence. Magnetic resonance imaging (MRI) of the brain revealed 7 × 4 × 5 cm large expansive lesion in left occipital lobe, anteriorly facing trigone of lateral ventricle with both cystic and necrotic structure, with marginal post-contract imbibition. Additional diffuse leptomeningeal imbibition was noticed. The child underwent gross resection of the tumor. Pathohistology report revealed anaplastic ependymoma, grade 3. Postoperative MRI depicted 3 × 4 × 4 cm large surgical cavity, with increased marginal signal on FLAIR sequence and intense post contrast imbibition indicating residual tumor with largest diameter of 2.2 cm. No malignant cells were found in cerebrospinal fluid. Chemotherapy according to E HIT 2000 protocol was initiated and patient received four cycles, before she was taken into the operating room for maximal re-resection. Following surgical recovery, the patient received course of highly conformal proton radiation therapy with total dose of 59.4 GyE in 33 daily fractions of 1.8 GyE each, 5 fractions per week. All treatments were delivered under general anesthesia. The 250 MeV cyclotrons were utilized for generating the proton beam. Clinical target volume (CTV) of 54 GyE encompassed the area of residual tumor and the resection cavity while CTV of 59.4 GyE encompassed the area of residual tumor. An additional 5 mm expansion formed the planning target volume (PTV). A high-resolution planning computerized tomography (CT) obtained in prone position was fused and registered with MRI for accurate definition of target volumes and the adjacent critical dose-limiting anatomical structures (brain parenchyma, brain stem, hippocampus, pituitary, optic nerves, chiasm). Dose distribution and the treatment plan was generated using 3D-treatment planning system (PSI-Plan), with integrated multiplanar isodose distribution and dose-volume histograms aiming to optimize the treatment plan.
After 6 months of disease-free period the patient recurred in the surgical cavity and intraventricular chemotherapy was initiated. Unfortunately, subsequent MRI revealed further progression of recurrent disease with additional 3 cm large de-novo metastasis in the right pontocerebellar angle. Also, new lesion was observed at S1 and S2 level of spinal canal measuring 2.3 cm which correspond to metastatic seeding deposit. Salvage ICE systemic chemotherapy was initiated however disease further progressed with development of new cortical metastasis in left temporal lobe measuring 1.2 cm along with progression of disease in sacral spinal canal now extending to L5 vertebrae and measuring 4 cm in longitudinal diameter.
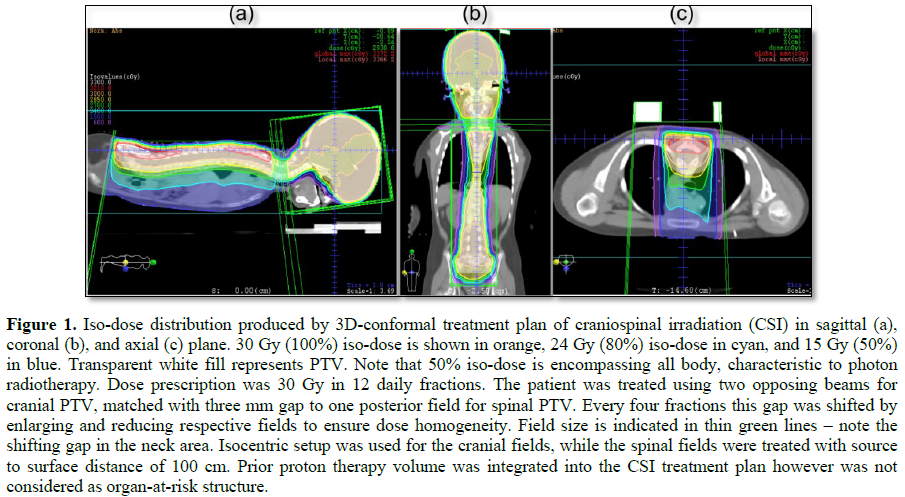
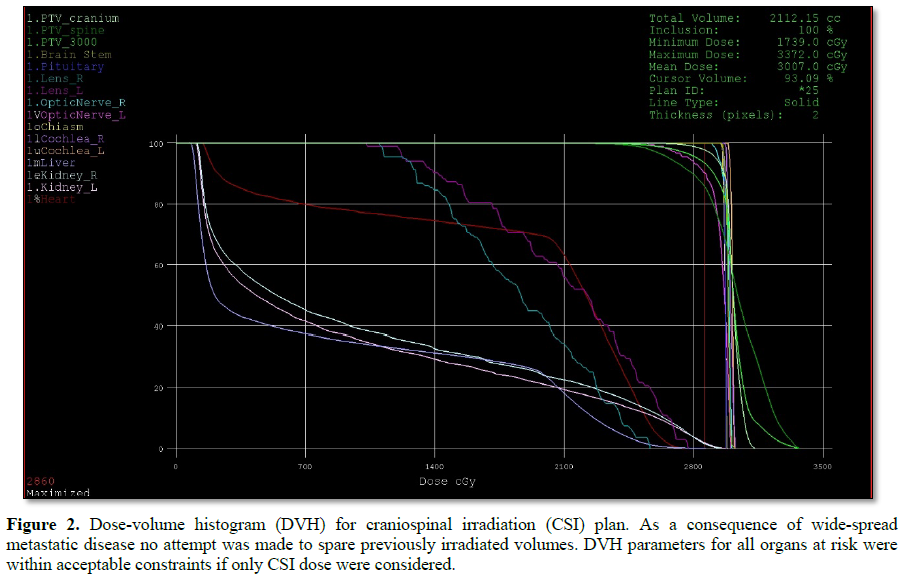
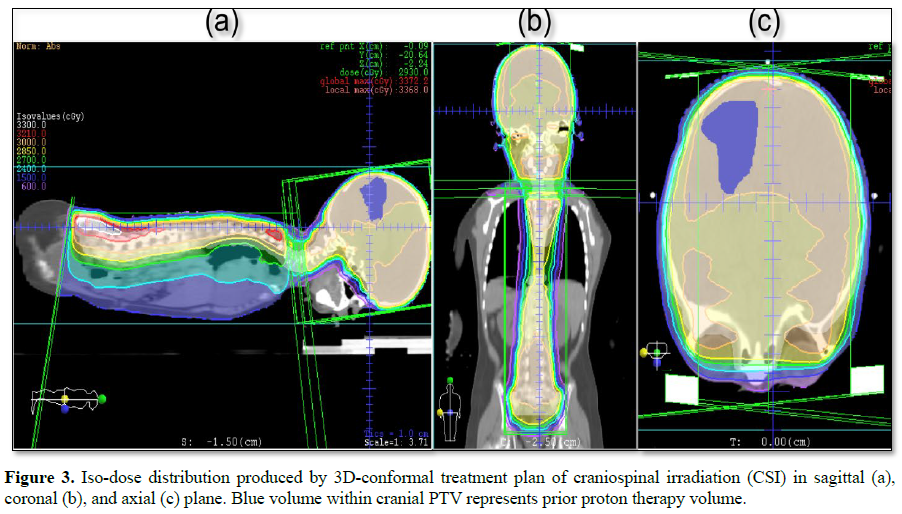
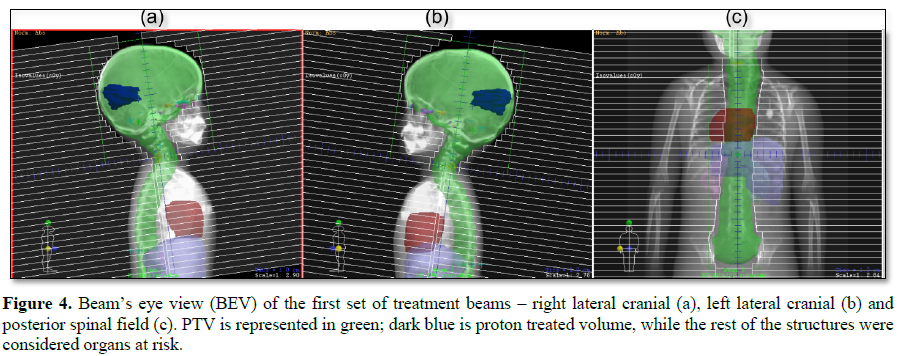
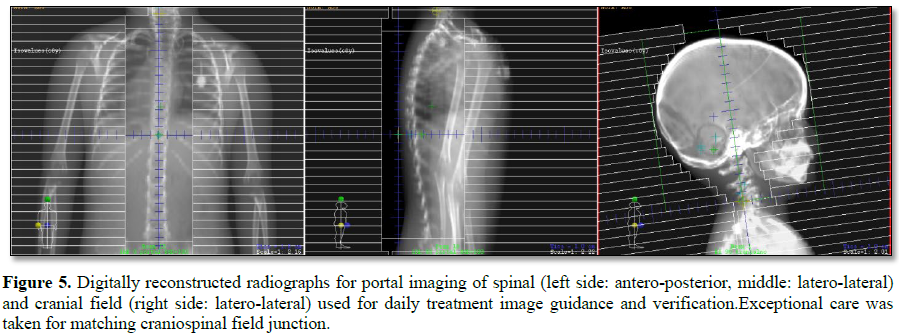
This challenging patient was discussed on pediatric oncology rounds and the patient was referred to palliative radiotherapy. No chemotherapy options remained for this patient. Given metastatic involvement of several CNS segments and neurologic deterioration decision was made to treat whole craniospinal axis with photon radiotherapy to a total dose of 30 Gy given in 12 daily fractions. 3D-conformal plan was generated with no effort to spare previous high-dose proton radiotherapy field given overt disease involvement (Figures 1-4). The patient was treated in prone position with daily portal imaging as method of image guidance and beam verification (Figure 5). Treatment was well tolerated and mild improvement in neurological condition was observed. However, within 3 months after radiotherapy patient experienced further intracranial, spinal progression and clinical deterioration and finally deceased 9 months after the treatment. Of note, no signs of myelopathy were observed on follow-up MRI post radiotherapy treatment.
DISCUSSION
The management of recurrent and metastatic ependymoma is very challenging and usually combines surgery, chemotherapy and radiotherapy. Despite multimodal treatment the clinical outcome is regularly poor and survival short. There is specifically lack of data regarding the use of radiotherapy in the post-proton therapy setting. However, given sharp dose fall-off characteristic for proton beam [5], one can assume improved tolerance of surrounding critical structures potentially translating to improved therapeutic ratio of salvage photon radiotherapy.
To best of our knowledge, we are not aware of any other report of salvage craniospinal photon beam irradiation after primary focal proton radiotherapy failure. On the other hand, some data exist on proton re-irradiation of previously proton-irradiated craniospinal axis or posterior fossa to a full dose showing feasibility of this approach [6,7]. Planning and treatment techniques were developed which enabled complete sparing of previously full-dose-irradiated surrounding critical structures [6].
Despite reirradiation to a palliative, though significant dose, it is encouraging to observe that our patient did not experience radiation myelitis or necrosis, typical radiation-related late toxicity in this setting. Although proton beam is considered to be highly conformal, with theoretically no exit dose, the concern for serious toxicity (mainly brain stem necrosis) stems from unknown effects of uncertain relative biological effectiveness of the proton beam on the brainstem [8]. However, in this patient, proton therapy CTV was distant from the brainstem thereby minimizing the risk of brain stem toxicity in subsequent re-irradiations, even taking into account worst case scenario that no interval repair occurred, which is less likely to happen [9].
Our patient lived 9 months after the craniospinal radiotherapy, which may be considered as long enough for this side-effect to be manifested [4].
To analyze the incidence of brain necrosis in patients who received craniospinal irradiation after primary focal cranial photon radiotherapy, we extrapolated data from landmark Merchanct study [3]. In this study, 19 patients, following surgical resection received craniospinal irradiation either for local, metastatic or combined failure. Among these 19 patients, only one developed necrosis (incidence 5%) at the site of initial focal treatment, 6 months after his primary radiotherapy to the fourth ventricle. This patient experienced metastatic progression in lumbosacral space. Craniospinal radiotherapy was delivered to the dose of 39.6 Gy followed by boost to gross disease to 59.4 Gy. Location of his necrosis was in cerebellum, and cumulative total dose in that region equated to 99 Gy. This patient however endured permanent neurologic impairment but experienced no disease relapse 2 years post salvage craniospinal irradiation. From 4 patients who were treated with craniospinal irradiation for combined failure (both focal and metastatic, similarly to our patient), 3 experienced progression of disease 7-9 months post salvage treatment. This is in keeping with our observation form this case that combined failures are hardly salvageable.
One strategy to mitigate the late toxicity associated with second course of craniospinal radiotherapy is the use hyperfractionation, like in the report by Gupta et al. [10]. According to linear-quadratic model this approach might potentially reduce neural tissue late toxicity while achieving equivalent tumor dose compared to standard fractionation [11]. Authors treated medulloblastoma multisegmental metastatic relapse with 30 Gy in 30 fractions (1 Gy/fraction, two fractions daily) to the brain, and 36 Gy in 30fractions (1.2 Gy/fraction, two fractions daily) to the spine, with subsequent boosting of gross disease to total dose of 45 and 48 Gy in 40 fractions. This approach produced 18-months long clinical response with no neural late toxicity observed.
The use of proton therapy in pediatric population is constantly rising. From the inception of this novel therapy, it was justifiably predicted the utilization will be highest in pediatric tumors. However, the mainstay of proton treatment, both focal and craniospinal is in the primary treatment setting not palliative. Typical indications for proton beam radiotherapy in United States are primary treatment for gliomas, medulloblastomas and ependymomas [12]. On account of advantageous physical properties of proton beam, unmatched dosimetric sparring can be achieved in critical surrounding structures, while allowing full dose irradiation of the target volume. This is the key advantage of the proton beam over the photon beam therapy and can be fully exploited in childhood brain tumors.
Our decision to offer the course of palliative craniospinal irradiation in the context of overt metastatic disease and progressive neurological deterioration may be subject of discussion. This was very challenging clinical context with no published data to provide guidance. Referral to child hospice was an option, however, by providing this hypofractionated treatment the short-term aim of palliation of growing obstructive hydrocephalus and neurological deterioration was achieved without causing severe neurological damage as evidenced both clinically and by MRI.
Recurrent ependymoma remains disease with huge unmet need in pediatric population where new ways of optimal integration of local therapies and systemic treatments should be explored.
1. Bouffet E, Hawkins CE, Ballourah W, Taylor MD, Bartels UK, et al. (2012) Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys 83: 1541-1548.
2. Cage TA, Clark AJ, Aranda D, Gupta N, Sun PP, et al. (2013) A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. J Neurosurg Pediatr 11: 673-681.
3. Merchant TE, Boop FA, Kun LE, Sanford RA (2008) A retrospective study of surgery and re-irradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 71: 87-97.
4. Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70: 1350-1360.
5. Newhauser WD, Zhang R (2015) The physics of proton therapy. Phys Med Biol 60: R155-R209.
6. McDonald MW, Wolanski MR, Simmons JW, Buchsbaum JC (2013) Technique for sparing previously irradiated critical normal structures in salvage proton craniospinal irradiation. Radiat Oncol 8.
7. Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, et al. (2015) Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 116: 301-308.
8. Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, et al. (2002) Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol 53: 407-421.
9. Nieder C, Milas L, Ang KK (2000) Tissue tolerance to re-irradiation. Semin Radiat Oncol 10: 200-229.
10. Gupta T, Nair V, Phurailatpam R, Jalali R, Sarin R (2012) Hyperfractionated craniospinal re-irradiation for recurrent/progressive disseminated medulloblastoma using image-guided radiotherapy: Leveraging radiobiology with technology. J Radiat Oncol 1: 87-92.
11. Supe S, Ganesh K, Naveen T, Jacob S, Sankar B (2006) Spinal cord response to altered fractionation and re-irradiation: Radiobiological considerations and role of bioeffect models. J Cancer Res Ther 2: 105.
12. Chang AL, Yock TI, Mahajan A, Hill-Kaiser C, Keole S, Loredo L, et al. (2014) Pediatric proton therapy: patterns of care across the United States. Int J Part Ther 1: 357-367.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)